Introduction
Tryptophol, an indole derivative with a wide array of potential benefits, has become a focal point in scientific research and industrial applications. This compound, notable for its presence in fermentation processes and its sedative properties, offers promising avenues in pharmaceuticals, cosmetics, and agriculture.
In this comprehensive guide, we will delve into the intricate methods of synthesizing tryptophol, explore its multifaceted applications, and provide expert advice on sourcing high-quality tryptophol for your research or industrial needs.
Whether you are a researcher seeking to understand its biochemical significance or a professional aiming to harness its potential, this article will equip you with the essential knowledge and practical insights needed to leverage tryptophol effectively.
Identification
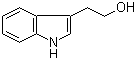
| Name | Tryptophol |
| Synonyms | beta-3-Indolylethanol; 3-(2-Hydroxyethyl)indole; 2-(1H-Indol-3-yl)ethan-1-ol |
| Molecular Formula | C10H11NO |
| Molecular Weight | 161.20 |
| CAS No. | 526-55-6 |
| EC No. | 208-393-2 |
Properties & Safety Data
| Melting point | 57-60 ºC |
| Water solubility | 10 g/L (20 ºC) |
| Safety data | S24/25 |
Our Specifications
| Appearance | Off-white or yellowish brown or reddish brown solid |
| Loss on drying | ≤ 0.5% |
| Melting Point | 57.0~60.0℃ |
| Assay (HPLC) | ≥ 98.0% |
| Package | 1Kg,5Kg,10Kg,20Kg per bag |
| Standard | In-house Standard |
The Applications of Tryptophol
Tryptophol's unique structure and properties make it suitable for a variety of applications in research, medicine, and industry. Here are some notable uses of tryptophol:
Biological Research
Tryptophol is widely used in biological research due to its role in various physiological processes. It is a product of tryptophan metabolism and can be studied to understand metabolic pathways and the effects of metabolic intermediates.
Applications:
- Investigating tryptophan metabolism
- Studying the effects of indole compounds on cellular processes
- Exploring tryptophol's role in sleep and mood regulation
Pharmaceuticals
The sedative and hypnotic properties of tryptophol have piqued interest in its potential therapeutic applications. Researchers are exploring its use in developing new medications for sleep disorders and anxiety.
Applications:
- Potential treatment for insomnia
- Development of anti-anxiety medications
- Research on neuroprotective effects
Food and Beverage Industry
Tryptophol naturally occurs in fermented foods and beverages, such as wine and beer, contributing to their relaxing effects. It is also studied for its potential health benefits when consumed as part of a diet.
Applications:
- Analyzing the presence and effects of tryptophol in fermented products
- Enhancing the health benefits of fermented foods
- Developing functional foods with added tryptophol
Cosmetics
The antioxidant properties of tryptophol make it a valuable ingredient in skincare products. It can help in protecting the skin from oxidative stress and promoting a youthful appearance.
Applications:
- Formulating anti-aging skincare products
- Developing treatments for skin inflammation
- Enhancing the protective effects of sunscreens
Agricultural Research
In plants, tryptophol acts as a growth regulator, influencing various developmental processes. Agricultural researchers study tryptophol to improve crop yields and resilience.
Applications:
- Studying plant growth regulation
- Developing agricultural treatments to enhance crop growth
- Investigating the effects of tryptophol on plant stress responses
How to Synthesize Tryptophol: A Complete Guide
Researchers have developed several synthesis methods to obtain pure tryptophol efficiently and cost-effectively.

Materials and Equipment Needed

Chemicals Required
List of chemicals
To synthesize tryptophol, specific chemicals are necessary. The primary chemicals include:
- Indole: A commercially available starting material.
- (lH-indol-3yl)-oxo-acetyl chloride: Used in the preparation process.
- Ethyl ester: Another key component in the synthesis.
- 4-Hydroxy butyraldehyde: Involved in the intermediate steps.
- o-Ethyl phenylhydrazine hydrochloride: Reacts with 4-hydroxy butyraldehyde.
Sources and suppliers
Obtaining high-quality chemicals ensures the success of the synthesis process. Reliable sources and suppliers include:
- ChemicalBook: Offers various synthetic routes and high-purity chemicals.
- Sigma-Aldrich: Provides a wide range of laboratory-grade chemicals.
- Fisher Scientific: Supplies essential reagents and solvents.
Equipment Required
List of equipment
The synthesis of tryptophol requires specific laboratory equipment. Essential items include:
- Continuous flow reactor: Facilitates the synthesis process.
- Tubular reactor: Used for continuous flow synthesis.
- Glassware: Beakers, flasks, and pipettes for handling chemicals.
- Heating mantle: Maintains required temperatures during reactions.
- Magnetic stirrer: Ensures uniform mixing of reactants.
Setup and preparation
Proper setup and preparation of equipment are crucial. Follow these steps:
- Assemble the continuous flow reactor: Ensure all connections are secure.
- Prepare the tubular reactor: Set the desired flow rates and temperatures.
- Arrange glassware: Organize beakers, flasks, and pipettes for easy access.
- Calibrate the heating mantle: Set the temperature according to the synthesis requirements.
- Position the magnetic stirrer: Place it under the reaction vessel for efficient mixing.
Simple Synthesis Method
Step-by-step procedure
- Prepare the reactants: Measure indole and 4-hydroxy butyraldehyde.
- Mix the reactants: Combine indole and 4-hydroxy butyraldehyde in a reaction vessel.
- Heat the mixture: Use a heating mantle to maintain the temperature at 80°C.
- Add a catalyst: Introduce a small amount of acid catalyst to the mixture.
- Stir continuously: Use a magnetic stirrer to ensure uniform mixing.
- Monitor the reaction: Observe the color change indicating the formation of tryptophol.
- Cool the mixture: Allow the reaction vessel to cool to room temperature.
- Isolate the product: Use a separating funnel to extract tryptophol from the reaction mixture.
Chemical reactions involved
The primary reaction involves the condensation of indole with 4-hydroxy butyraldehyde. The acid catalyst facilitates this reaction, leading to the formation of tryptophol.
Required temperatures and solvents
- Temperature: Maintain at 80°C during the reaction.
- Solvents: Use methanol as the solvent for the reaction.
Intermediate Synthesis Method
Step-by-step procedure
- Dissolve indole: Use a glycol-ether solvent to dissolve indole.
- Add 4-hydroxy butyraldehyde: Introduce 4-hydroxy butyraldehyde to the solution.
- Heat the solution: Set the temperature to 100°C using a heating mantle.
- Introduce o-ethyl phenylhydrazine hydrochloride: Add this reagent to the mixture.
- Stir the mixture: Ensure thorough mixing with a magnetic stirrer.
- Maintain the temperature: Keep the reaction at 100°C for 2 hours.
- Cool the mixture: Allow the reaction vessel to cool down gradually.
- Separate the product: Use a separating funnel to isolate tryptophol.
Chemical reactions involved
The intermediate method involves a two-step reaction. First, indole reacts with 4-hydroxy butyraldehyde. Then, the resulting compound reacts with o-ethyl phenylhydrazine hydrochloride.
Required temperatures and solvents
- Temperature: Maintain at 100°C during the reaction.
- Solvents: Use glycol-ether as the solvent.
Advanced Synthesis Method
Step-by-step procedure
- Set up a continuous flow reactor: Assemble the reactor and ensure all connections are secure.
- Introduce indole and PHH: Feed indole and PHH into the reactor.
- Control the flow rate: Adjust the flow rate to achieve optimal mixing.
- Heat the reactor: Maintain the temperature at 120°C.
- Monitor the reaction: Use sensors to track the progress of the reaction.
- Collect the product: Continuously collect tryptophol from the reactor outlet.
- Purify the product: Use chromatography to purify the collected tryptophol.
Chemical reactions involved
The advanced method employs a continuous flow process. Indole and PHH react in a tubular reactor, forming tryptophol efficiently.
Required temperatures and solvents
- Temperature: Maintain at 120°C during the reaction.
- Solvents: Use an environmentally benign solvent like methanol.
Safety Precautions

Handling Chemicals Safely
Personal protective equipment (PPE)
Laboratory personnel must wear appropriate personal protective equipment (PPE) to ensure safety. Essential PPE includes:
- Lab coat: Protects skin and clothing from chemical splashes.
- Safety goggles: Shields eyes from harmful chemicals and reactions.
- Gloves: Prevents direct contact with hazardous substances.
- Face mask: Reduces inhalation of toxic fumes and particles.
Safe storage and disposal
Proper storage and disposal of chemicals minimize risks. Follow these guidelines:
- Label containers: Clearly mark all chemical containers with contents and hazards.
- Store chemicals properly: Keep chemicals in designated storage areas, away from incompatible substances.
- Dispose of waste correctly: Use appropriate waste containers for chemical disposal. Follow local regulations for hazardous waste management.
- Maintain an inventory: Regularly update the chemical inventory to track usage and storage.
Laboratory Safety
Ventilation and fume hoods
Effective ventilation and use of fume hoods are crucial for maintaining a safe laboratory environment. Key practices include:
- Use fume hoods: Conduct all reactions involving volatile or hazardous chemicals inside fume hoods.
- Ensure proper airflow: Regularly check fume hoods for adequate airflow and functionality.
- Avoid overcrowding: Do not block vents or overfill fume hoods with equipment and chemicals.
- Monitor air quality: Install air quality monitors to detect harmful fumes and ensure a safe working environment.
Emergency procedures
Preparedness for emergencies can save lives and prevent injuries. Implement these procedures:
- Know emergency exits: Familiarize yourself with all emergency exits and evacuation routes.
- Locate safety equipment: Identify the locations of fire extinguishers, eyewash stations, and safety showers.
- Report incidents immediately: Inform supervisors and safety officers of any accidents or spills.
- Conduct regular drills: Participate in emergency drills to practice evacuation and response procedures.
By following these safety precautions, laboratory personnel can minimize risks and ensure a safe working environment during the synthesis of tryptophol.
Troubleshooting Tips
Common Issues and Solutions
Reaction not proceeding
A common issue in synthesizing tryptophol involves the reaction not proceeding. Several factors can cause this problem. First, check the quality of the chemicals. Low-purity chemicals can hinder the reaction. Ensure that all reactants meet laboratory-grade standards.
Second, verify the temperature settings. Each synthesis method requires specific temperatures. For example, the simple synthesis method needs a temperature of 80°C. Use a calibrated heating mantle to maintain accurate temperatures.
Third, examine the mixing process. Inadequate mixing can prevent the reactants from interacting properly. Use a magnetic stirrer to ensure uniform mixing throughout the reaction.
Impurities in the product
Impurities in the final product can affect the quality of tryptophol. To address this issue, start by reviewing the reaction conditions. High temperatures or prolonged reaction times can introduce impurities. Follow the recommended conditions for each synthesis method.
Next, consider the solvents used. Impure solvents can introduce contaminants. Use high-purity solvents like methanol or glycol-ether to minimize impurities.
Finally, assess the purification process. Insufficient purification can leave residual impurities. Techniques like chromatography can help achieve a higher purity level.
Optimizing Yield
Adjusting reaction conditions
Optimizing yield involves adjusting reaction conditions. Start by fine-tuning the temperature. Each synthesis method has an optimal temperature range. For instance, the advanced synthesis method requires 120°C. Maintaining this temperature can improve yield.
Next, control the flow rate in continuous flow reactors. Adjusting the flow rate ensures optimal mixing and reaction time. This adjustment can enhance the efficiency of the synthesis process.
Lastly, consider the concentration of reactants. Higher concentrations can increase the reaction rate. However, avoid excessively high concentrations to prevent side reactions.
Purification techniques
Purification techniques play a crucial role in optimizing yield. One effective method involves using chromatography. This technique separates impurities from the desired product. Chromatography can significantly improve the purity and yield of tryptophol.
Another method involves recrystallization. Dissolve the crude product in a suitable solvent. Then, cool the solution to precipitate pure tryptophol. This process helps remove soluble impurities.
A developed process for synthesizing tryptophol achieved a 92% yield without additional purification. This high yield demonstrates the importance of optimizing reaction conditions and using effective purification techniques.
By addressing common issues and optimizing reaction conditions, the synthesis of tryptophol can achieve higher yields and purer products.
Variations of the Synthesis Process
Alternative Methods
Different starting materials
Different starting materials can lead to variations in the synthesis of tryptophol. One alternative involves using indole-3-acetic acid as a precursor. This method provides a different pathway for synthesizing tryptophol. Another approach uses 2,3-dihydrofuran (2,3-DHF) in combination with phenylhydrazine hydrochloride (PHH). This combination offers an efficient route for producing tryptophol in a continuous flow reactor.
Modified reaction conditions
Modified reaction conditions can enhance the synthesis process. Adjusting the temperature and solvent type can yield better results. For instance, using glycol-ether solvents instead of methanol can improve the reaction efficiency. Additionally, varying the reaction temperature can optimize the yield. A developed process for synthesizing tryptophol achieved high yields by maintaining specific temperature settings.
Potential Modifications
Scaling up the synthesis
Scaling up the synthesis process can meet industrial demands. Using larger reactors and increasing the quantity of starting materials can produce more tryptophol. Continuous flow reactors facilitate this scaling-up process. These reactors allow for the continuous introduction of reactants and collection of products. This method ensures a steady production rate and consistent quality.
Customizing the product
Customizing the product involves modifying the synthesis process to obtain specific derivatives of tryptophol. One example is the synthesis of 7-ethyltryptophol. This compound serves as a key intermediate in various pharmaceutical applications. Researchers have developed a simple and general method for synthesizing 7-ethyltryptophol. This method offers several advantages, including operational simplicity and high yield.
By exploring alternative methods and potential modifications, researchers can optimize the synthesis of tryptophol. These variations can lead to improved efficiency, higher yields, and customized products suitable for various applications.