Chiral Alcohols
Discover Reisch’s expertise in high-purity chiral alcohols for pharmaceuticals and chemicals. We offer custom synthesis, rigorous quality assurance, and secure handling of your projects, ensuring precision and reliability from R&D to large-scale production.
Products You May Be Interested In
CAS NO. 1517-69-7
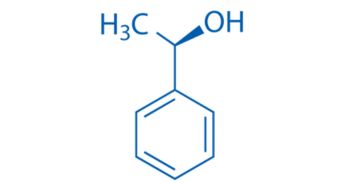
- 99%min
- Colorless to light yellow transparent liquid
- Boiling point: 88-89 °C/10 mmHg
- Storage: Sealed in dry, Room Temperature
- Package: 1Kg, 5Kg, 10Kg, 25Kg
CAS No. 1445-91-6
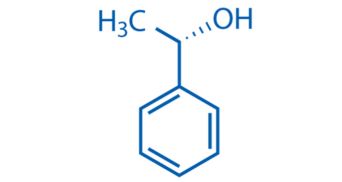
- 99%min
- Colorless transparent liquid
- Boiling point: 88-89 °C10 mm Hg(lit.)
- storage temp.: 2-8°C
- Pacakge: 1Kg, 5Kg, !0Kg, 25Kg
CAS NO. 171032-87-4
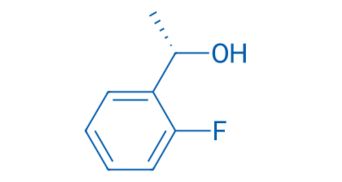
- 99%min
- Colorless transparent liquid
- Storage: Sealed in dry,Room Temperature
- Boiling point: 193℃
- Package: 1Kg, 5Kg, 10Kg, 25Kg
CAS NO. 19132-06-0
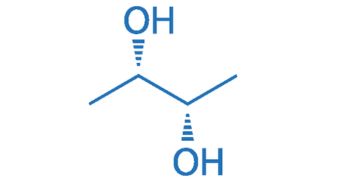
- 99%min
- Colorless transparent liquid
- Boiling point:179-182 °C (lit.)
- Keep in dark place, Inert atmosphere, Room temperature
- Package: 1Kg, 5Kg, 10Kg, 25Kg
CAS NO. 17299-07-9
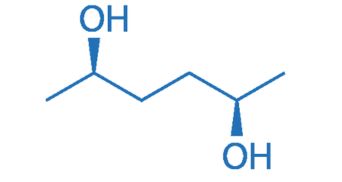
- 99%min
- White to yellow crystalline powder
- Melting point: 52-54 °C
- Storage: Inert atmosphere, Room Temperature
- Package: 1Kg, 5Kg, 10Kg, 25Kg
CAS NO. 42075-32-1
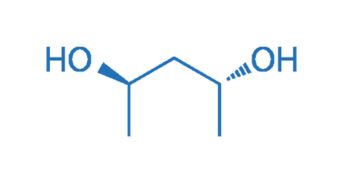
- 99%min
- White crystal
- Melting point: 48-50 °C(lit.)
- Storage: Inert atmosphere, Room Temperature
- Package: 1Kg, 5Kg, 10Kg, 25Kg
CAS NO. 72345-23-4
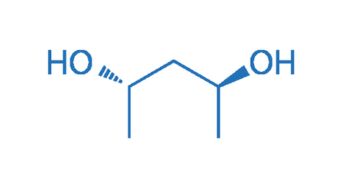
- 99%min
- White crystalline Powder
- Melting point:45-48 °C (lit.)
- Storage: Inert atmosphere, Room Temperature
- Package: 1Kg, 5Kg, 10Kg, 25Kg
CAS NO. 6290-03-5
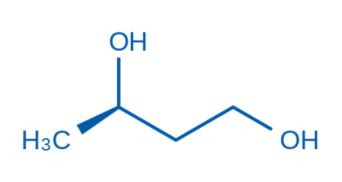
- 98%min
- Colorless transparent oil liquid
- Boiling point: 107-110 °C/23 mmHg (lit.)
- Storage: Inert atmosphere, Room Temperature
- Package: 1Kg, 5Kg, 10Kg, 25kg
CAS NO. 76116-24-0
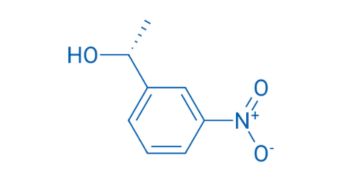
- 98%min
- Colorless transparent liquid
- Boiling point: 218℃
- Storage: Sealed in dry, 2-8°C
- Package: 1Kg, 5Kg, 10Kg, 20Kg
CAS NO. 76155-78-7
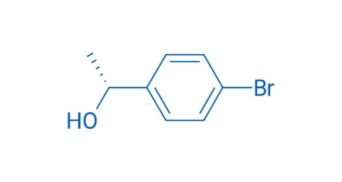
- 98%min
- Light yellow transparent liquid
- Boiling point: 110 °C(Press: 3.0 Torr)
- Storage: Sealed in dry, Room Temperature
- Package: 1Kg, 5Kg, 10Kg, 20Kg
CAS NO. 34338-96-0
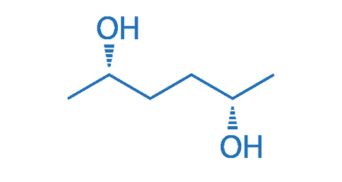
- 99%min
- White crystals
- Melting point: 50-53 °C(lit.)
- Storage: Inert atmosphere, Room Temperature
- Package: 1Kg, 5Kg, 10Kg, 20Kg
CAS NO. 24347-58-8
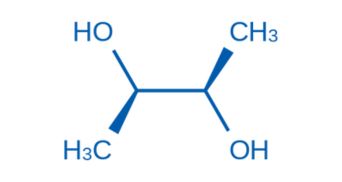
- 98%min
- Colorless transparent liquid
- Boiling point: 77.3-77.4 °C/10 mmHg (lit.)
- storage: Sealed in dry,Store in freezer, under -20°C
- Melting point: 16 °C
CAS NO. 25779-13-9
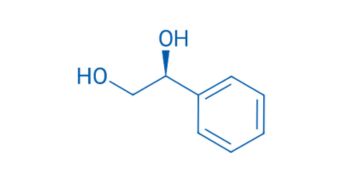
- 98%min
- Off-white crystalline powder
- Melting point: 64-67 °C(lit.)
- Boiling point: 272-274 °C (755 mmHg)
- Storage: Sealed in dry, Room Temperature
CAS NO. 131864-71-6
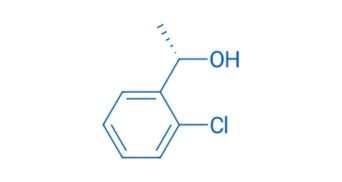
- 98%min
- Colorless transparent liquid
- Boiling point: 231℃
- Storage: Sealed in dry, Room Temperature
- Package: 1Kg, 5Kg, 10Kg, 20Kg
CAS NO. 135145-34-5

- 97%min
- Colorless transparent liquid
- Boiling point: 230℃
- Storage: Sealed in dry, 2-8°C
- Package: Based on custmer
CAS NO. 5096-11-7

- 98%min
- Colorless transparent liquid
- Boiling point: 80 °C(Press: 1.5 Torr)
- Storage: Inert atmosphere, 2-8°C
- Package: Based on customer
CAS NO. 126534-31-4

- 98%min
- Colorless transparent liquid
- Boiling point: 323.3±37.0 °C(Predicted)
- Storage: Sealed in dry, 2-8°C
- Package: based on order
Contact us to discuss your chiral alcohol needs
Please provide the CAS number, product name, quantity, and any special requirements you have. With this information, we will promptly respond with detailed solutions and a comprehensive quote. Contact us now to get started!
Chiral Alcohols: Essential Intermediates in Chemical Synthesis
Chiral alcohols play a crucial role in the realm of chemical synthesis, particularly in the pharmaceutical and fine chemical industries. These compounds, characterized by their unique three-dimensional structures, are indispensable intermediates in the production of a wide range of chiral molecules, which are often necessary for creating effective and safe drugs.
Table of Contents
Importance of Chiral Alcohols
Chirality is a property of asymmetry where a molecule has a non-superimposable mirror image. This characteristic is vital in the pharmaceutical industry because the two enantiomers (mirror images) of a chiral molecule can have drastically different effects in biological systems. One enantiomer might be therapeutically beneficial, while the other could be inactive or even harmful. Therefore, the production of enantiomerically pure compounds is essential for drug development and manufacturing.
Chiral alcohols are used as building blocks or intermediates in the synthesis of these enantiomerically pure compounds. Their ability to influence the stereochemistry of the molecules they help create makes them invaluable in the design and production of pharmaceuticals, agrochemicals, and fine chemicals.
Synthesis of Chiral Alcohols
The synthesis of chiral alcohols can be achieved through various methods, each chosen based on the desired end product’s complexity and requirements. Some of the common methods include:
Asymmetric Reduction:
Enzymatic or chemical reduction of ketones or aldehydes to produce chiral alcohols. This method often employs chiral catalysts to ensure high enantioselectivity.
Asymmetric Catalysis:
Using chiral ligands in transition metal-catalyzed reactions to introduce chirality into the alcohol.
Biocatalysis:
Utilizing enzymes such as lipases, esterases, or dehydrogenases to catalyze the formation of chiral alcohols with high specificity and under mild conditions.
Resolution of Racemates:
Separating racemic mixtures (equal parts of both enantiomers) into individual enantiomers through physical or chemical methods, such as crystallization or chromatography.


Applications in the Pharmaceutical Industry
The pharmaceutical industry relies heavily on chiral alcohols for the synthesis of active pharmaceutical ingredients (APIs). These intermediates are used to create complex molecules with precise three-dimensional structures necessary for drug efficacy and safety. For example, many antiviral, anticancer, and cardiovascular drugs are synthesized using chiral alcohol intermediates.
In addition to pharmaceuticals, chiral alcohols find applications in the production of agrochemicals, where the selective action of chiral pesticides and herbicides can be exploited to enhance effectiveness and reduce environmental impact.

Quality and Purity Considerations
The production of chiral alcohols demands high levels of purity and enantiomeric excess to ensure the desired pharmacological effects.
Advanced analytical techniques, such as nuclear magnetic resonance (NMR), high-performance liquid chromatography (HPLC), and gas chromatography (GC), are employed to characterize and verify the purity and stereochemistry of these compounds.
Reisch's Commitment to Excellence
At Reisch, we specialize in the custom synthesis of high-purity chiral alcohols tailored to meet the specific needs of our clients in the pharmaceutical and chemical industries. Our state-of-the-art facilities and expert team ensure that we deliver products of the highest quality, adhering to stringent regulatory standards.
Our services include:
Custom Synthesis
Tailored solutions for the production of specific chiral alcohols.
Quality Assurance
Rigorous testing and validation to ensure purity and enantiomeric excess.
Confidentiality
Secure handling of client information and intellectual property.
By leveraging advanced synthetic techniques and a deep understanding of stereochemistry, Reisch provides reliable and efficient solutions for the production of chiral alcohols. Whether for research and development or large-scale manufacturing, our commitment to quality and precision ensures that our clients receive the best possible products and services.
Contact Reisch today to learn more about how our expertise in chiral alcohol synthesis can support your pharmaceutical and chemical projects. Together, we can achieve your goals with precision and reliability.
Common Questions
Most Popular Questions
We maintain rigorous quality control procedures and adhere to the highest standards of quality and certification. Our commitment to uncompromising quality is a fundamental part of our business philosophy.
We provide continuous communication, technical assistance, and timely delivery of services, ensuring a seamless and professional collaboration experience.
We serve pharmaceutical and innovative companies, focusing on delivering high-quality chemical processes, control, and manufacturing services.
Our dedication to excellence, continuous improvement, and adherence to our core values make us a reliable partner in the pharmaceutical and healthcare industries.
Our products are known for their high purity, stability, and customization options to meet specific customer requirements.
Our customers include large pharmaceutical manufacturers, generic drug manufacturers, biotechnology companies, CMOs, CROs, academic and research institutions, government and military organizations, and specialty chemical companies.
Please contact our sales team through our website or email us at [email protected]. Provide details about your requirements, and we will respond with a tailored quote.
You can stay updated by visiting our website regularly and following our social media channels for the latest news and updates.
Payment terms vary based on the specific agreement with each client. We accept bank transfers (T/T), Letters of Credit (L/C), and other methods as agreed upon during contract negotiations. Details will be provided in your contract.
Products are shipped via reliable freight carriers, ensuring timely and safe delivery. We offer various shipping options, including air and sea freight, depending on your location and urgency. Delivery times depend on the destination and shipping method chosen. We will provide an estimated delivery timeline when your order is confirmed.
Shipping costs vary based on the order size, destination, and shipping method. These costs will be included in your quote and discussed during the order confirmation process.
Once your order is shipped, we will provide you with tracking information so you can monitor the progress of your shipment.
If there are any delays, please contact our customer service team immediately. We will investigate the issue and provide you with an update as soon as possible.
Additional fees, such as customs duties and import taxes, may apply depending on the destination country’s regulations. These fees are typically the responsibility of the recipient and will be communicated during the shipping process.